1 月 . 29, 2025 05:55
Back to list
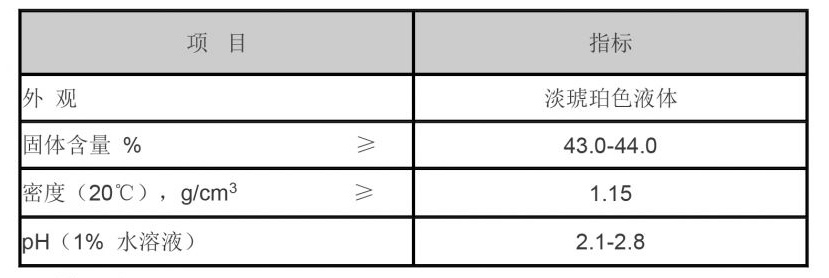
Diethylene Triamine Penta (Methylene Phosphonic Acid)(DTPMPA)
Polyacrylamide, a polymer formed from acrylamide subunits, has emerged as a key component in industries ranging from water treatment to enhanced oil recovery. Understanding its formation is critical to harnessing its full potential, particularly in product development and application-based scenarios.
Real-world experience in dealing with the nuances of polyacrylamide formation is invaluable. Field studies and pilot projects provide insights that bench-scale experiments may overlook. For example, the scale-up of polyacrylamide production from laboratory to industrial scale can present challenges such as heat management and uniformity, requiring skilled engineers to devise solutions that maintain the integrity of the polymer structure. Trust in polyacrylamide products is built through stringent quality control processes and transparent reporting. Manufacturers employ a combination of spectroscopic analysis and viscosity measurements to ensure that each batch conforms to expected quality standards. Documentation of these processes, along with third-party certifications and compliance reports, bolster consumer confidence in the product's reliability. Furthermore, continuous innovation in the synthesis and modification of polyacrylamide has led to the development of novel derivatives with superior properties. For instance, copolymerization with other monomers can impart functionalities such as thermosensitivity or biodegradability, expanding the range of applications in industries like agriculture and biomedicine. In conclusion, the formation of polyacrylamide is a complex interplay of chemistry and engineering that demands a high level of expertise. By prioritizing safety, quality, and regulatory compliance, manufacturers can produce high-performance polyacrylamide suited for diverse applications. Companies that lead in this space are those that invest in cutting-edge research and embrace sustainable practices, ensuring that their products remain at the forefront of technological advancement. As the demand for polyacrylamide continues to grow, those with authoritative knowledge and hands-on experience in its production will be best positioned to capitalize on emerging market opportunities.

Real-world experience in dealing with the nuances of polyacrylamide formation is invaluable. Field studies and pilot projects provide insights that bench-scale experiments may overlook. For example, the scale-up of polyacrylamide production from laboratory to industrial scale can present challenges such as heat management and uniformity, requiring skilled engineers to devise solutions that maintain the integrity of the polymer structure. Trust in polyacrylamide products is built through stringent quality control processes and transparent reporting. Manufacturers employ a combination of spectroscopic analysis and viscosity measurements to ensure that each batch conforms to expected quality standards. Documentation of these processes, along with third-party certifications and compliance reports, bolster consumer confidence in the product's reliability. Furthermore, continuous innovation in the synthesis and modification of polyacrylamide has led to the development of novel derivatives with superior properties. For instance, copolymerization with other monomers can impart functionalities such as thermosensitivity or biodegradability, expanding the range of applications in industries like agriculture and biomedicine. In conclusion, the formation of polyacrylamide is a complex interplay of chemistry and engineering that demands a high level of expertise. By prioritizing safety, quality, and regulatory compliance, manufacturers can produce high-performance polyacrylamide suited for diverse applications. Companies that lead in this space are those that invest in cutting-edge research and embrace sustainable practices, ensuring that their products remain at the forefront of technological advancement. As the demand for polyacrylamide continues to grow, those with authoritative knowledge and hands-on experience in its production will be best positioned to capitalize on emerging market opportunities.
Share
Latest news
-
The Ultimate Guide to Flocculants: Transforming Water TreatmentNewsNov.01,2024
-
Improve Your Water Treatment Solutions with PolyacrylamideNewsNov.01,2024
-
Enhance Your Water TreatmentNewsNov.01,2024
-
Empower You to Achieve the Highest Standards of Water QualityNewsNov.01,2024
-
Effective Scale InhibitorsNewsNov.01,2024
-
Discover the Power of Poly Aluminum Chloride in Water TreatmentNewsNov.01,2024





