2 月 . 14, 2025 04:55
Back to list
cas 40372-66-5
CAS Number 40372-66-5, known scientifically as ropivacaine, is an amide-type local anesthetic used widely in medical and surgical settings for its prolonged analgesic effect and favorable side effect profile. Understanding the distinctive characteristics and practical applications of ropivacaine can greatly enlighten both medical professionals and entities involved in pharmaceutical manufacturing.
Trustworthiness in the application of ropivacaine is underscored by global regulatory endorsements, including approvals by the FDA and EMA, affirming its consistent performance and safety in various anesthetic modalities. Healthcare institutions reporting active usage often cite improved patient satisfaction ratings, a metric increasingly significant in the domain of health services. The data supporting ropivacaine's lower incidence of adverse events compared to its counterparts reinforces the trust healthcare providers place in this anesthetic solution. On the production front, manufacturers of ropivacaine must adhere to stringent quality control measures, contributing to its esteemed status in pharmaceuticals. Its synthesis demands precision in organic chemistry, ensuring that each batch meets high standards necessary for patient safety and efficacy. An understanding of the intricate formulation processes helps pharmaceutical companies maintain a significant degree of expertise in their production pipelines. In conclusion, ropivacaine’s innovative formulation not only elevates its standing as a superior anesthetic agent but also positions it as a cornerstone in modern anesthesia practices. The compound's proven benefits in sensory block specificity, patient safety, and operational efficiency represents a compelling case for its inclusion in diverse therapeutic settings, advocating a shift towards safer, more effective pain management solutions.
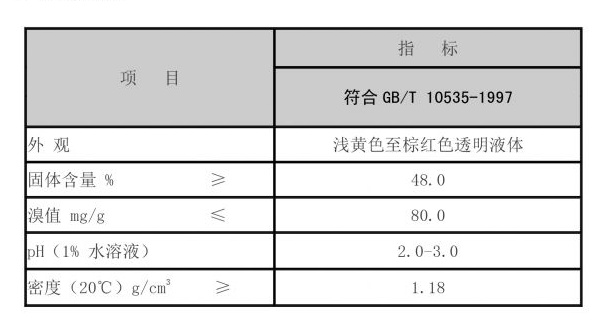

Trustworthiness in the application of ropivacaine is underscored by global regulatory endorsements, including approvals by the FDA and EMA, affirming its consistent performance and safety in various anesthetic modalities. Healthcare institutions reporting active usage often cite improved patient satisfaction ratings, a metric increasingly significant in the domain of health services. The data supporting ropivacaine's lower incidence of adverse events compared to its counterparts reinforces the trust healthcare providers place in this anesthetic solution. On the production front, manufacturers of ropivacaine must adhere to stringent quality control measures, contributing to its esteemed status in pharmaceuticals. Its synthesis demands precision in organic chemistry, ensuring that each batch meets high standards necessary for patient safety and efficacy. An understanding of the intricate formulation processes helps pharmaceutical companies maintain a significant degree of expertise in their production pipelines. In conclusion, ropivacaine’s innovative formulation not only elevates its standing as a superior anesthetic agent but also positions it as a cornerstone in modern anesthesia practices. The compound's proven benefits in sensory block specificity, patient safety, and operational efficiency represents a compelling case for its inclusion in diverse therapeutic settings, advocating a shift towards safer, more effective pain management solutions.
Share
Next:
Latest news
-
The Ultimate Guide to Flocculants: Transforming Water TreatmentNewsNov.01,2024
-
Improve Your Water Treatment Solutions with PolyacrylamideNewsNov.01,2024
-
Enhance Your Water TreatmentNewsNov.01,2024
-
Empower You to Achieve the Highest Standards of Water QualityNewsNov.01,2024
-
Effective Scale InhibitorsNewsNov.01,2024
-
Discover the Power of Poly Aluminum Chloride in Water TreatmentNewsNov.01,2024





